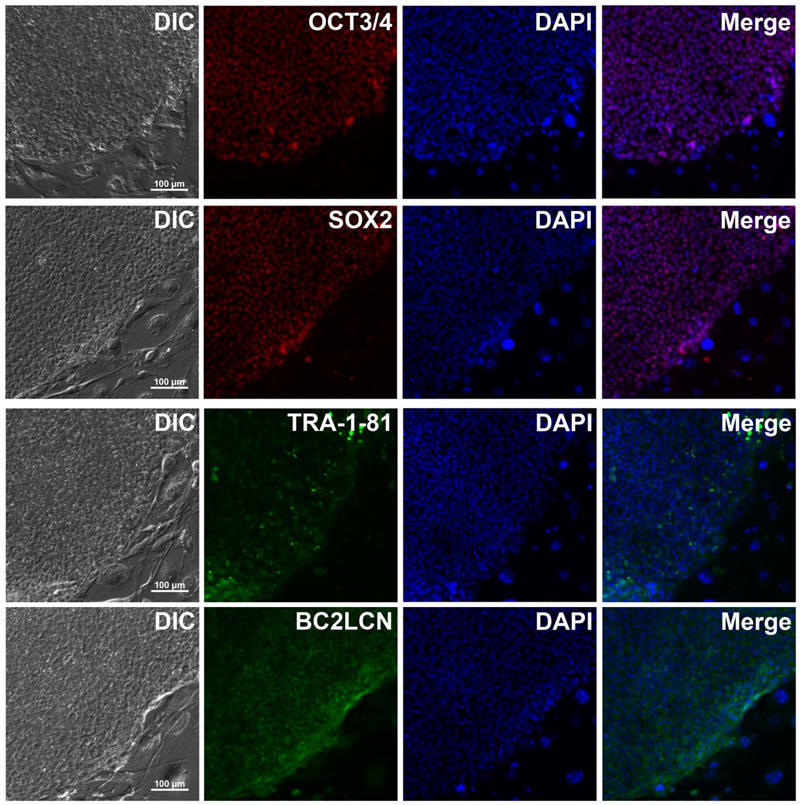
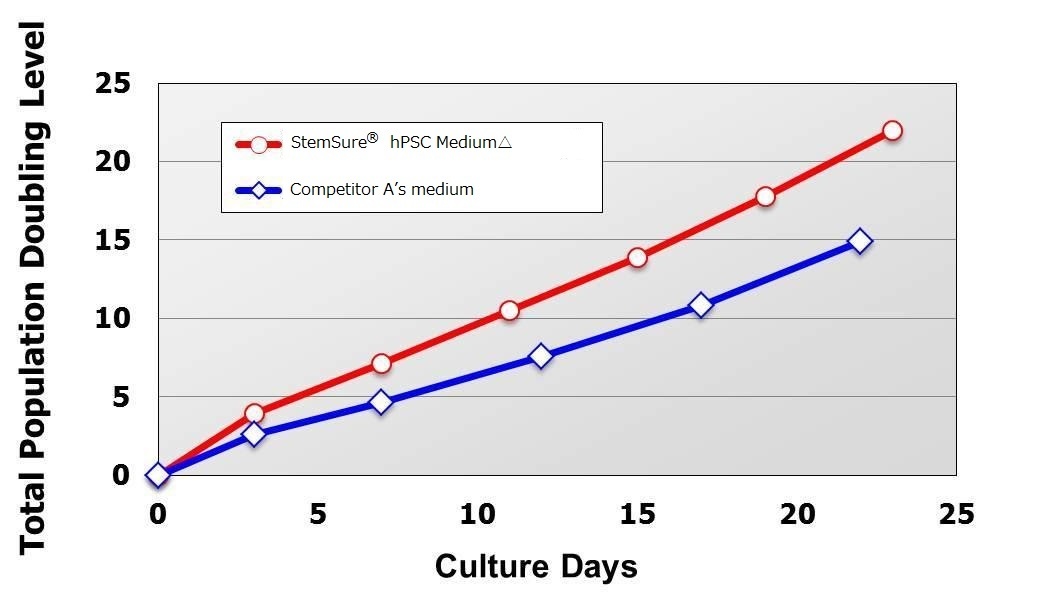
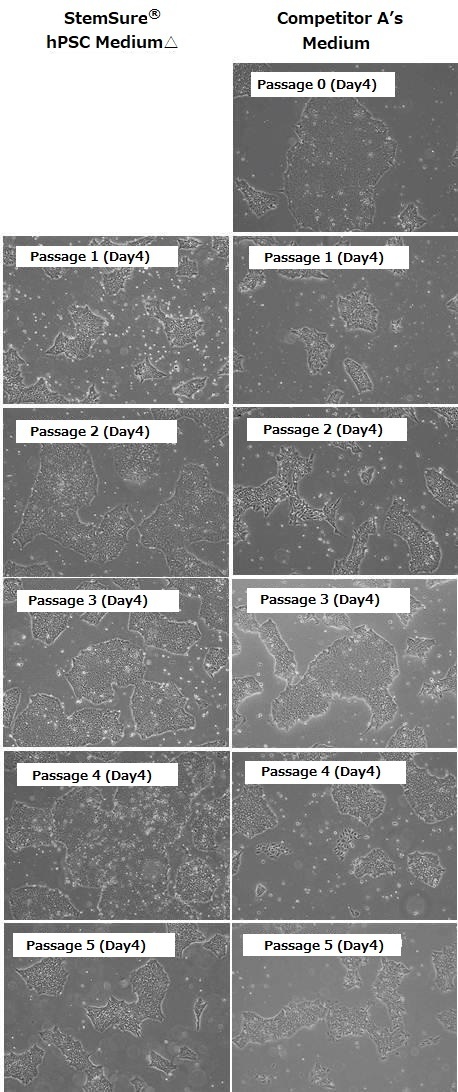
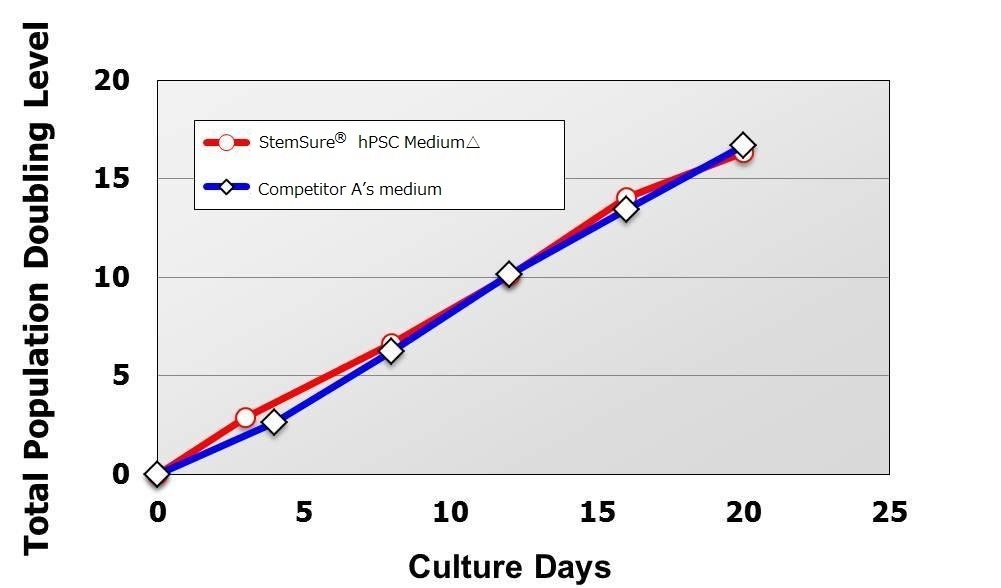
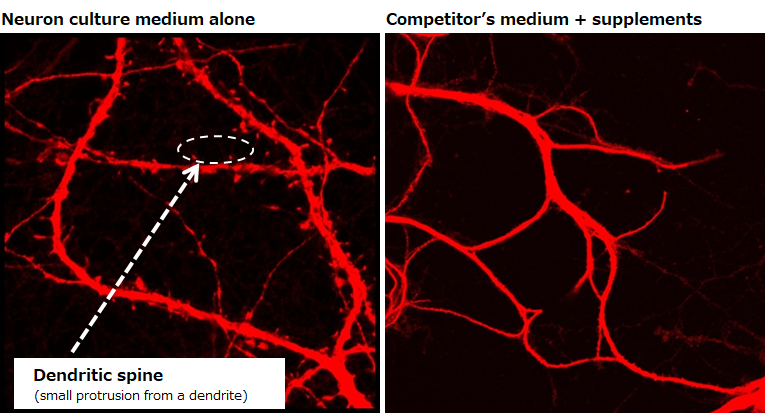
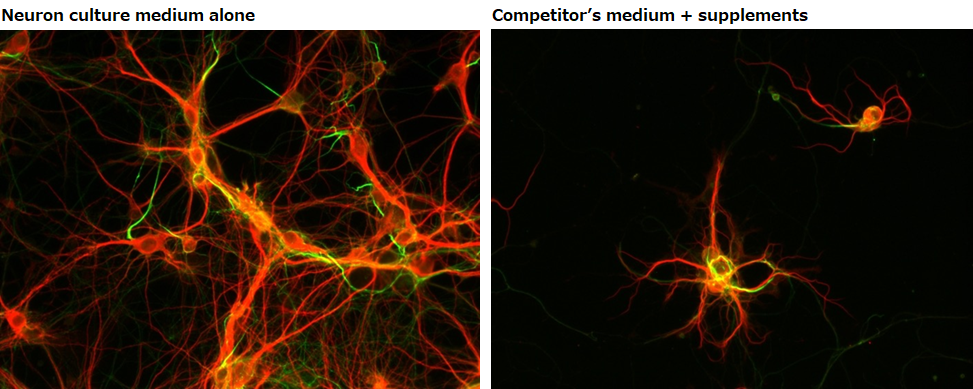
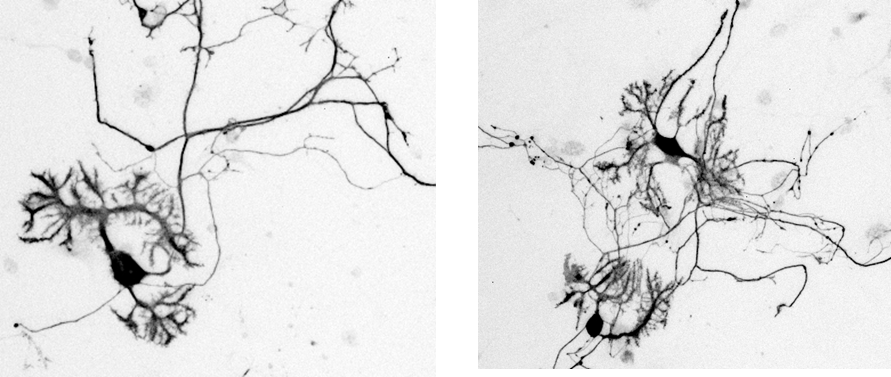
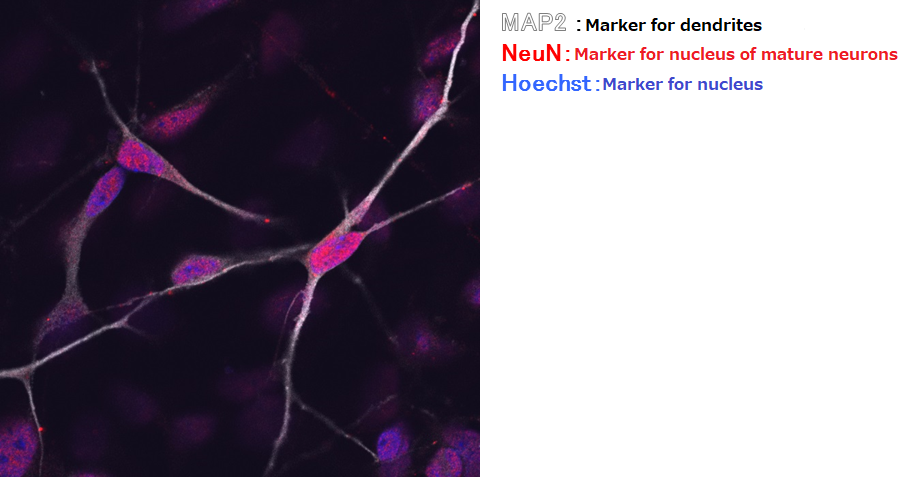
Rat, Recombinant Cytokines
We have a variety of rat recombinant cytokines and growth factors. Please contact our distributors for the pricing and estimated delivery time of bulk orders.
- Directions for use
- What are animal-free cytokines?
- Q&A
- Product List
- Related Product List
- Related Information
Directions for use
The following steps describe the use of freeze-dried products. Products available as solutions are ready-to-use.
- Centrifuge the product (e.g. using a tabletop centrifuge) for 20-30 sec before opening the lid.
- Dissolve in a buffer as described in the package insert. Dilute into a buffer or cell culture medium to be used in the experiment. Do not vortex.
- Keep the product in the fridge if it is to be used within a week. If you are storing for a longer time period, add the carrier proteins and make small batches of the product for each use to avoid multiple thawing and freezing. Freeze the batches of the product for future use.
What are animal-free cytokines?
Animal-free cytokines are expressed and purified from E. coli that was cultured without any animal-derived materials. There is a minimal risk of viral contaminations that would otherwise be caused by the presence of animal-derived raw materials. There cytokines can be used in the same way as regular cytokines.
Features
- Cytokines were expressed in E. coli .
- No animal-derived materials were used during the E. coli culturing and purification processes (certificates are available).
- The products have been sterilized by filtration and are freeze-dried.
- The products have undergone change control processes.
Q&A
Q1. I cannot see proteins in the tube clearly. Is there anything in it?
Proteins are included in the product as indicated. It may be difficult to see the small amount of proteins by eye as most of the products do not contain carrier proteins. Proteins may also be stuck on the lid or the sides of the tube. Please make sure to centrifuge the tube (for 20-30 sec) prior to use.
Q2. The proteins look different from the previous product that I used. Is there a problem? (Looks like white powder or translucent gel)
Proteins may appear differently even for the same product due to its volume, composition, and the state of freeze-drying. However, there have been no reports to date that suggest that such difference has any impact on the quality of the product.
Q3. How do I thaw freeze-dried products?
The best protocol for thawing is described in the insert for each product.
Solubility of proteins may be affected if methods other than those described are used the first time you thaw the product (e.g. using buffers that contain carrier proteins). Incomplete dissolution may result in poor activity.
Q4. How do I store freeze-dried products after thawing?
Keep in the fridge if you are going to use all of the product within 1 week after thawing. For long-term storage, add a carrier protein and make small batches of the product for each use to avoid multiple thawing and freezing.
Q5. Which carrier proteins should I use?
We recommend the use of 0.1% BSA for regular products and 5% trehalose for animal-free products.
Q6. Are freeze-dried products sterilized?
The products are sterilized by filtration with 0.2 μm filter prior to freeze-dry.
Q7. What do terms “ED50” and “specific activity” that are used in the product insert under biological activity mean?
ED50 is short for effective dose 50, and is defined as 50% of the concentration of cytokines needed to achieve its maximum biological activity. The dose-response curve for cytokines would have a sigmoid shape in this case. Specific activity is calculated as ED50 per unit amount of a protein (units/mg).
Q8. What are animal-free cytokines?
Animal-free cytokines are expressed and purified from E. coli that was cultured without any animal-derived materials. There is a minimal risk of viral contaminations that would otherwise be caused by the presence of animal-derived raw materials. There cytokines can be used in the same way as regular cytokines.
Product List
- Open All
- Close All
Related Product List
- Open All
- Close All
Buffer / Carrier protein
※Use for the adjustment of cytokines.。
Related Information
Category
- Cell Culture
- Animal Cell Culture
- Cytokine / Growth Factor
- Cell Culture
- Stem Cell Culture
- Cytokine / Growth Factor
- CultureSure® Small Molecules
- Mouse, Recombinant Cytokines
- Human, Recombinant Cytokines
Product content may differ from the actual image due to minor specification changes etc.