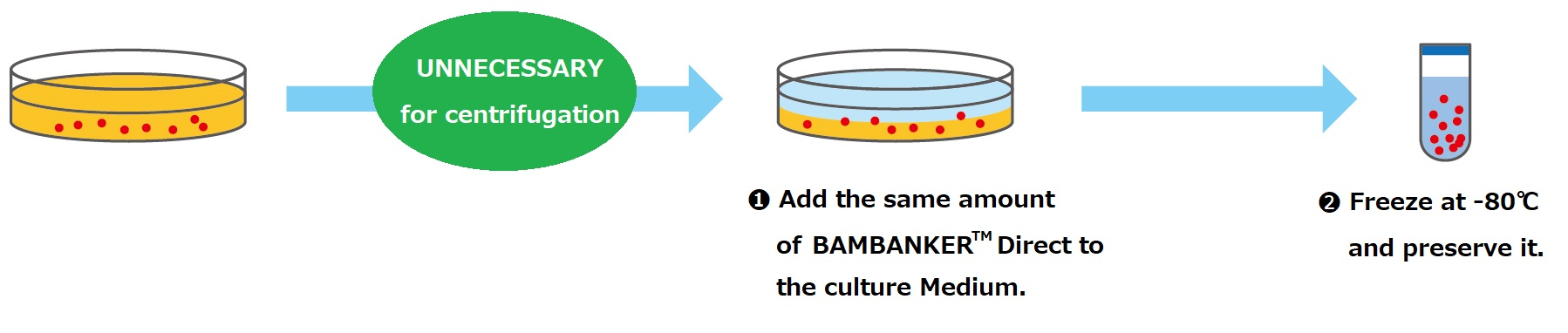
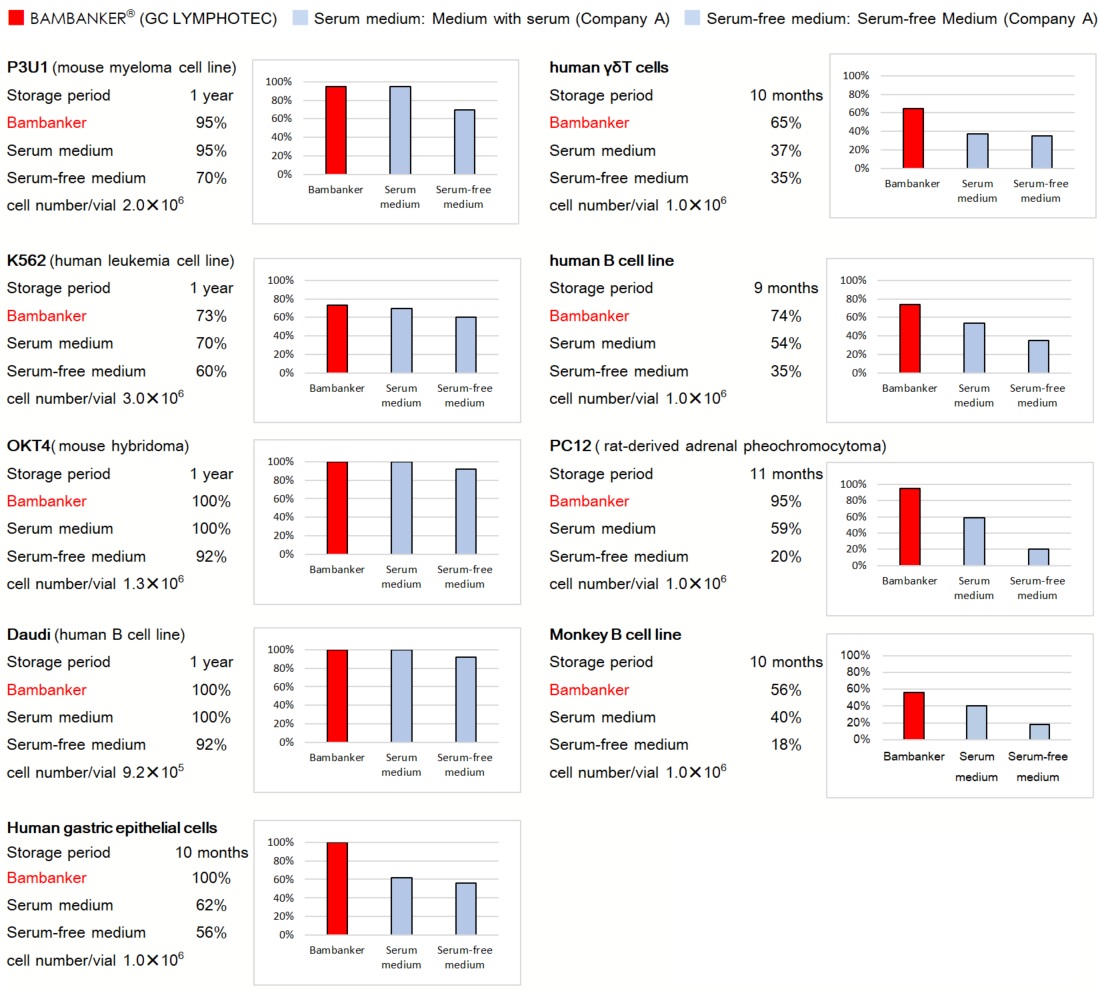
CultureSure® 10w/v% Polyoxyethylene Polyoxypropylene Glycol Solution
This product is a 10% aqueous solution of a nonionic surfactant designed for cell culture applications.
In insect cell cultures, it is used as a media additive to both suppress cell aggregation and prevent cell attachment to flasks.
While acting as an antifoaming agent, this surfactant limits the interaction of cells with air bubbles, thus protecting cells against injury. It also defends cells from shear forces produced by sparging air. The beneficial effects of this surfactant have been shown to promote cell proliferation.
- Product Information
- References
- Product List
- Related Product List
- Related Information
Product Information
- Liquid format
- Endotoxin: ≦0.5 EU/mL
- Sterility tested
- Mycoplasma tested
References
- David W. M. and Charles F. G.: BIO/TECHNOLOGY, 6, 1411(1988).
- Jordan M. et al: Biotechnol Bioeng. , 43, 446(1994).
- Gilbert R. S. et al: Cytotechnology, 22, 211(1996).
Product List
- Open All
- Close All
Related Product List
- Open All
- Close All
Liquid culture medium for insect culture
Medium additive reagent
Related Information
Category
- Cell Culture
- Animal Cell Culture
- Cell Preservation
Product content may differ from the actual image due to minor specification changes etc.